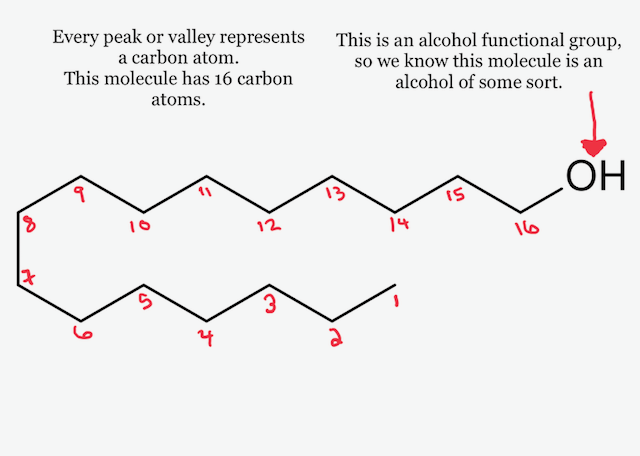
#alltheingredients: TKB Thickening clay – part three, version 1
The suggestion and recipes shared by TKB Trading for this thickening clay didn’t work for me, so I thought I’d go on a quest for other formulas I could try at home to succeed with this ingredient. I found a formula that called for fractionated coconut oil and isopropyl myristate (IPM) as the oils with 3%...