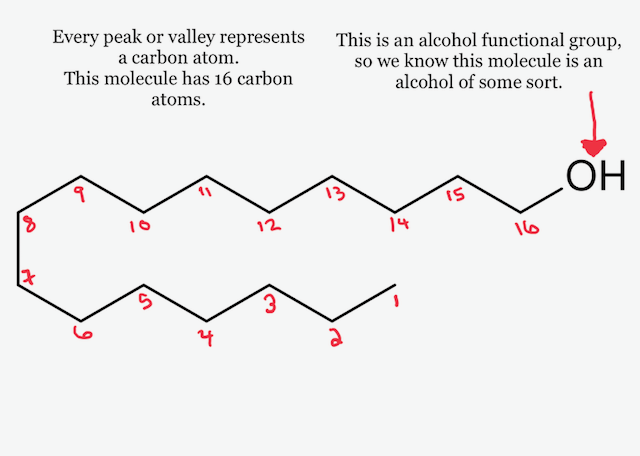
#alltheingredients Isododecane
Isododecane is a hydrocarbon alkane with no double bonds. It’s a non-polar, oil soluble molecule that only contains carbon and hydrogen atoms. We can use it as an oily ingredient anywhere we might use natural oils or butters (vegetable, seed, and animal oils), as well as with esters and silicones. It’s considered an isoparaffin. More...